-
(환경) Methane (영문)과학과 테크놀로지/환경 2016. 1. 9. 01:35
출처: https://en.wikipedia.org/wiki/Methane
Methane
From Wikipedia, the free encyclopedia"CH4" redirects here. For other uses, see CH4 (disambiguation).For the emergency service protocol, see ETHANE.Methane 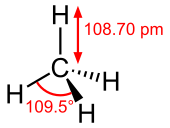
Names IUPAC name Other names - Carbane
- Marsh Gas
- Natural Gas
- Carbon tetrahydride
- Hydrogen carbide
Identifiers 74-82-8 
3DMet B01450 1718732 ChEBI CHEBI:16183 
ChEMBL ChEMBL17564 
ChemSpider 291 
EC Number 200-812-7 59 Jmol interactive 3D Image KEGG C01438 
MeSH Methane PubChem 297 RTECS number PA1490000 UN number 1971 Properties CH4 Molar mass 16.04 g·mol−1 Appearance Colorless gas Odor Odorless Density 0.656 g/L (gas, 25 °C, 1 atm)
0.716 g/L (gas, 0 °C, 1 atm)
0.42262 g cm−3
(liquid, −162 °C)[2]Melting point −182.5 °C; −296.4 °F; 90.7 K Boiling point −161.49 °C; −258.68 °F; 111.66 K 22.7 mg L−1 Solubility soluble in ethanol, diethyl ether, benzene, toluene,methanol, acetone log P 1.09 Henry's law
constant (kH)14 nmol Pa−1 kg−1 Structure Td Tetrahedron 0 D Thermochemistry 35.69 J K−1 mol−1 Std molar
entropy (So298)186.25 J K−1 mol−1 Std enthalpy of
formation(ΔfHo298)−74.87 kJ mol−1 Std enthalpy of
combustion(ΔcHo298)−891.1 to −890.3 kJ mol−1 Hazards[3] Safety data sheet See: data page GHS pictograms 
GHS signal word DANGER H220 P210 EU classification(DSD) F+
F+R-phrases R12 S-phrases (S2), S16, S33 NFPA 704 Flash point −188 °C (−306.4 °F; 85.1 K) 537 °C (999 °F; 810 K) Explosive limits 4.4–17% Related compounds Related alkanesSupplementary data page Refractive index (n),
Dielectric constant (εr), etc.Thermodynamic
dataPhase behaviour
solid–liquid–gasUV, IR, NMR, MS  verify (what is
verify (what is 
 ?)
?)Infobox references Methane (/ˈmɛθeɪn/ or /ˈmiːθeɪn/) is a chemical compound with the chemical formula CH4 (one atom of carbon and four atoms ofhydrogen). It is the simplest alkane and the main component of natural gas. The relative abundance of methane on Earth makes it an attractive fuel, though capturing and storing it poses challenges due to its gaseous state found at standard conditions for temperature and pressure.
In its natural state, methane is found both below ground and under the sea floor, where it often finds its way to the surface and the atmosphere where it is known as atmospheric methane.[5] The Earth's atmospheric methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases (these gases don't include water vapor which is by far the largest component of the greenhouse effect).[6]Methane breaks down in the atmosphere and creates CH·3 with water vapor.
Contents
[hide]History
![[icon]](https://upload.wikimedia.org/wikipedia/commons/thumb/1/1c/Wiki_letter_w_cropped.svg/20px-Wiki_letter_w_cropped.svg.png)
This section requires expansion.(November 2013) In November 1776, methane was first scientifically identified by Italian physicist Alessandro Volta in the marshes of Lake Maggiorestraddling Italy and Switzerland, having been inspired to search for the substance after reading a paper written by Benjamin Franklin about "flammable air".[7] Volta captured the gas rising from the marsh, and by 1778 had isolated the pure gas.[8] He also demonstrated means to ignite the gas with an electric spark.[8]
Properties and bonding
Methane is a tetrahedral molecule with four equivalent C-H bonds. Its electronic structure is described by four bonding molecular orbitals (MOs) resulting from the overlap of the valence orbitals on C and H. The lowest energy MO is the result of the overlap of the 2s orbital on carbon with the in-phase combination of the 1s orbitals on the four hydrogen atoms. Above this level in energy is a triply degenerate set of MOs that involve overlap of the 2p orbitals on carbon with various linear combinations of the 1s orbitals on hydrogen. The resulting "three-over-one" bonding scheme is consistent with photoelectron spectroscopic measurements.
At room temperature and standard pressure, methane is a colorless, odorless gas.[9] The familiar smell of natural gas as used in homes is a safety measure achieved by the addition of an odorant, usually blends containing tert-butylthiol. Methane has a boiling point of −161 °C (−257.8 °F) at a pressure of one atmosphere.[10] As a gas it is flammable over a range of concentrations (4.4–17%) in air at standard pressure.
Solid methane exists in several modifications. Presently nine are known.[11] Cooling methane at normal pressure results in the formation of methane I. This substance crystallizes in the cubic system (space group Fm3m). The positions of the hydrogen atoms are not fixed in methane I, i.e., methane molecules may rotate freely. Therefore, it is a plastic crystal.[12]
Chemical reactions
Main reactions with methane are: combustion, steam reforming to syngas, and halogenation. In general, methane reactions are difficult to control. Partial oxidation to methanol, for example, is challenging because the reaction typically progresses all the way to carbon dioxide and water even with incomplete amounts of oxygen. The enzyme methane monooxygenase produces methanol from methane, but cannot be used for industrial-scale reactions.[13]
Acid-base reactions
Like other hydrocarbons, methane is a very weak acid. Its pKa in DMSO is estimated to be 56.[14] It cannot be deprotonated in solution, but the conjugate base with methyllithium is known.
A variety of positive ions derived from methane have been observed, mostly as unstable species in low-pressure gas mixtures. These include methenium or methyl cation CH+
3, methane cation CH+
4, and methanium or protonated methane CH+
5. Some of these have been detected in outer space. Methanium can also be produced as diluted solutions from methane with super acids. Cations with higher charge, such as CH2+
6 and CH3+
7, have been studied theoretically and conjectured to be stable.[15]Despite the strength of its C-H bonds, there is intense interest in catalysts that facilitate C–H bond activation in methane (and other low alkanes).[16]
Combustion
Methane's heat of combustion is 55.5 MJ/kg.[17] Combustion of methane is a multiple step reaction. The following equations are part of the process, with the net result being:
CH4 + 2 O2 → CO2 + 2 H2O (ΔH = −891 k J/mol (at standard conditions))
- CH4+ M* → CH3 + H + M
- CH4 + O2 → CH3 + HO2
- CH4 + HO2 → CH3 + 2 OH
- CH4 + OH → CH3 + H2O
- O2 + H → O + OH
- CH4 + O → CH3 + OH
- CH3 + O2 → CH2O + OH
- CH2O + O → CHO + OH
- CH2O + OH → CHO + H2O
- CH2O + H → CHO + H2
- CHO + O → CO + OH
- CHO + OH → CO + H2O
- CHO + H → CO + H2
- H2 + O → H + OH
- H2 + OH → H + H2O
- CO + OH → CO2 + H
- H + OH + M → H2O + M*
- H + H + M → H2 + M*
- H + O2 + M → HO2 + M*
The species M* signifies an energetic third body, from which energy is transferred during a molecular collision. Formaldehyde (HCHO or H
2CO) is an early intermediate (reaction 7). Oxidation of formaldehyde gives the formyl radical (HCO; reactions 8–10), which then give carbon monoxide (CO) (reactions 11, 12 & 13). Any resulting H2oxidizes to H2O or other intermediates (reaction 14, 15). Finally, the CO oxidizes, forming CO2 (reaction 16). In the final stages (reactions 17–19), energy is transferred back to other third bodies. The overall speed of reaction is a function of the concentration of the various entities during the combustion process. The higher the temperature, the greater the concentration of radical species and the more rapid the combustion process.[18]Reactions with halogens
Methane reacts with halogens given appropriate conditions as follows:
- X2 + UV → 2 X•
- X• + CH4 → HX + CH3•
- CH3• + X2 → CH3X + X•
where X is a halogen: fluorine (F), chlorine (Cl), bromine (Br), or iodine (I). This mechanism for this process is called free radical halogenation. It is initiated with UV light or some other radical initiator. A chlorine atom is generated from elemental chlorine, which abstracts a hydrogen atom from methane, resulting in the formation of hydrogen chloride. The resulting methyl radical, CH3•, can combine with another chlorine molecule to give methyl chloride (CH3Cl) and a chlorine atom. This chlorine atom can then react with another methane (or methyl chloride) molecule, repeating the chlorination cycle.[19] Similar reactions can produce dichloromethane (CH2Cl2), chloroform (CHCl3), and, ultimately, carbon tetrachloride (CCl4), depending upon reaction conditions and the chlorine to methane ratio.
Uses
Methane is used in industrial chemical processes and may be transported as a refrigerated liquid (liquefied natural gas, or LNG). While leaks from a refrigerated liquid container are initially heavier than air due to the increased density of the cold gas, the gas at ambient temperature is lighter than air. Gas pipelines distribute large amounts of natural gas, of which methane is the principal component.
Fuel
Methane can be used as a gas for ovens, hobs etc. It combusts with oxygen to create fire.
Natural gas
Main article: natural gasMethane is important for electrical generation by burning it as a fuel in a gas turbine or steam generator. Compared to other hydrocarbon fuels, burning methane produces less carbon dioxide for each unit of heat released. At about 891 kJ/mol, methane's heat of combustion is lower than any other hydrocarbon but the ratio of the heat of combustion (891 kJ/mol) to the molecular mass (16.0 g/mol, of which 12.0 g/mol is carbon) shows that methane, being the simplest hydrocarbon, produces more heat per mass unit (55.7 kJ/g) than other complex hydrocarbons. In many cities, methane is piped into homes for domestic heating and cooking purposes. In this context it is usually known as natural gas, which is considered to have an energy content of 39 megajoules per cubic meter, or 1,000 BTU per standard cubic foot.
Methane in the form of compressed natural gas is used as a vehicle fuel and is claimed to be more environmentally friendly than other fossil fuels such as gasoline/petrol anddiesel.[20] Research into adsorption methods of methane storage for use as an automotive fuel has been conducted.[21]
Liquefied natural gas
Main article: Liquefied natural gasLiquefied natural gas (LNG) is natural gas (predominantly methane, CH4) that has been converted to liquid form for ease of storage or transport.
Liquefied natural gas takes up about 1/600th the volume of natural gas in the gaseous state. It is odorless, colorless, non-toxic and non-corrosive. Hazards include flammability after vaporization into a gaseous state, freezing and asphyxia.
The liquefaction process involves removal of certain components, such as dust, acid gases, helium, water, and heavy hydrocarbons, which could cause difficulty downstream. The natural gas is then condensed into a liquid at close to atmospheric pressure (maximum transport pressure set at around 25 kPa or 3.6 psi) by cooling it to approximately −162 °C (−260 °F).
LNG achieves a higher reduction in volume than compressed natural gas (CNG) so that the energy density of LNG is 2.4 times greater than that of CNG or 60% that of diesel fuel.[22] This makes LNG cost efficient to transport over long distances where pipelines do not exist. Specially designed cryogenic sea vessels (LNG carriers) or cryogenic road tankers are used for its transport.
LNG, when it is not highly refined for special uses, is principally used for transporting natural gas to markets, where it is regasified and distributed as pipeline natural gas. It is also beginning to be used in LNG-fueled road vehicles. For example, trucks in commercial operation have been achieving payback periods of approximately four years on the higher initial investment required in LNG equipment on the trucks and LNG infrastructure to support fueling.[23] However, it remains more common to design vehicles to usecompressed natural gas. As of 2002, the relatively higher cost of LNG production and the need to store LNG in more expensive cryogenic tanks had slowed widespread commercial use.[24]
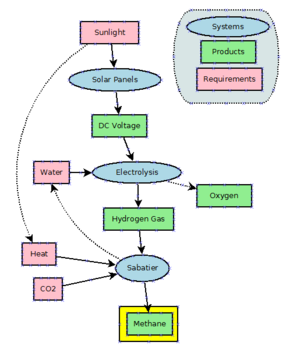
Power to gas
Main article: Power to gasPower to gas is a technology which converts electrical power to a gas fuel. The method is used to convert carbon dioxide and water to methane, (see natural gas) usingelectrolysis and the Sabatier reaction.[clarification needed] The excess power or off peak power generated by wind generators or solar arrays could theoretically be used for load balancing in the energy grid.[citation needed]
Liquid methane rocket fuel
In a highly refined form, liquid methane is used as a rocket fuel.[25]
While investigations of methane use have existed for decades, no production methane engines have yet been used on orbital spaceflights.[26] This is changing, and liquid methane has recently been selected for the active development of a variety of bipropellant rocket engines.
Since the 1990s, a number of Russian rockets have been proposed to use liquid methane.[27][28] One 1990s Russian engine proposal was the RD-192, a methane/LOX variant of the RD-191.[28]
In 2005, US companies, Orbitech and XCOR Aerospace, developed a demonstration liquid oxygen/liquid methane rocket engine and a larger 7,500 pounds-force (33 kN)-thrust engine in 2007 for potential use as the CEV lunar return engine, before the CEV program was later cancelled.[29][30][31]
More recently the American private space company SpaceX announced in 2012 an initiative to develop liquid methane rocket engines,[32] including, initially, the very largeRaptor rocket engine.[33] Raptor is being designed to produce 4.4 meganewtons (1,000,000 lbf) of thrust with a vacuum specific impulse (Isp) of 363 seconds and a sea-level Isp of 321 seconds,[34] and is expected to begin component-level testing in 2014.[35] In February 2014, the Raptor engine design was revealed to be of the highly efficient and theoretically more reliable full-flow staged combustion cycle type, where both propellant streams—oxidizer and fuel—will be completely in the gas phase before they enter the combustion chamber. Prior to 2014, only two full-flow rocket engines have ever progressed sufficiently to be tested on test stands, but neither engine completed development or flew on a flight vehicle.[34]
In October 2013, the China Aerospace Science and Technology Corporation, a state-owned contractor for the Chinese space program, announced that it had completed a first ignition test on a new LOX methane rocket engine. No engine size was provided.[36]
In September 2014, another American private space company—Blue Origin— publically announced that they were into their third year of development work on a large methane rocket engine. The new engine, the Blue Engine 4, or BE-4, has been designed to produce 2,400 kilonewtons (550,000 lbf) of thrust. While initially planned to be used exclusively on a Blue Origin proprietary launch vehicle, it will now be used on a new United Launch Alliance (ULA) engine on an new launch vehicle that is a successor to the Atlas V. ULA indicated in 2014 that they will make the maiden flight of the new launch vehicle no earlier than 2019.[37]
One advantage of methane is that it is abundant in many parts of the solar system and it could potentially be harvested on the surface of another solar-system body (in particular, using methane production from local materials found on Mars[38] or Titan), providing fuel for a return journey.[25][39]
By 2013, NASA's Project Morpheus had developed a small restartable LOX methane rocket engine with 5,000 pounds-force (22 kN) thrust and a specific impulse of 321 seconds suitable for inspace applications including landers. Small LOX methane thrusters 5–15 pounds-force (22–67 N) were also developed suitable for use in a Reaction Control System (RCS).[40][41]
SpaceNews is reporting in early 2015 that the French space agency CNES is working with Germany and a few other governments and will propose a LOX/methane engine on a reusable launch vehicle by mid-2015, with flight testing unlikely before approximately 2026.[42]
Chemical feedstock
Although there is great interest in converting methane into useful or more easily liquefied compounds, the only practical processes are relatively unselective. In the chemical industry, methane is converted to synthesis gas, a mixture of carbon monoxide and hydrogen, by steam reforming. This endergonic process (requiring energy) utilizes nickelcatalysts and requires high temperatures, around 700–1100 °C:
- CH4 + H2O → CO + 3 H2
Related chemistries are exploited in the Haber-Bosch Synthesis of ammonia from air, which is reduced with natural gas to a mixture of carbon dioxide, water, and ammonia.
Methane is also subjected to free-radical chlorination in the production of chloromethanes, although methanol is a more typical precursor.[43]
Production
Biological routes
Main article: methanogenesisNaturally occurring methane is mainly produced by the process of methanogenesis. This multistep process is used by microorganisms as an energy source. The net reaction is:
- CO2 + 8 H+ + 8 e− → CH4 + 2 H2O
The final step in the process is catalyzed by the enzyme Coenzyme-B sulfoethylthiotransferase. Methanogenesis is a form of anaerobic respiration used by organisms that occupy landfill, ruminants (e.g., cattle), and the guts of termites.
It is uncertain if plants are a source of methane emissions.[44][45][46]
Serpentinization
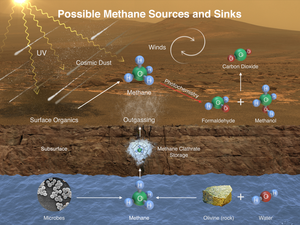
Methane could also be produced by a non-biological process called serpentinization[a] involving water, carbon dioxide, and the mineral olivine, which is known to be common on Mars.[47]
Industrial routes
Methane can be produced by hydrogenating carbon dioxide through the Sabatier process. Methane is also a side product of the hydrogenation of carbon monoxide in the Fischer-Tropsch process. This technology is practiced on a large scale to produce longer chain molecules than methane.
Natural gas is so abundant that the intentional production of methane is relatively rare. The only large scale facility of this kind is the Great Plains Synfuels plant, started in 1984 in Beulah, North Dakota as a way to develop abundant local resources of low grade lignite, a resource which is otherwise very hard to transport for its weight, ashcontent, low calorific value and propensity to spontaneous combustion during storage and transport.
An adaptation of the Sabatier methanation reaction may be used via a mixed catalyst bed and a reverse water gas shift in a single reactor to produce methane from the raw materials available on Mars, utilizing water from the Martian subsoil and carbon dioxide in the Martian atmosphere.[38]
Laboratory synthesis
Methane can also be produced by the destructive distillation of acetic acid in the presence of soda lime or similar. Acetic acid is decarboxylated in this process. Methane can also be prepared by reaction of aluminium carbide with water or strong acids.
Occurrence
Methane was discovered and isolated by Alessandro Volta between 1776 and 1778 when studying marsh gas from Lake Maggiore. It is the major component of natural gas, about 87% by volume. The major source of methane is extraction from geological deposits known as natural gas fields, with coal seam gas extraction becoming a major source (see Coal bed methane extraction, a method for extracting methane from a coal deposit, while enhanced coal bed methane recovery is a method of recovering methane from non-mineable coal seams). It is associated with other hydrocarbon fuels, and sometimes accompanied by helium and nitrogen. The gas at shallow levels (low pressure) forms by anaerobic decay of organic matter and reworked methane from deep under the Earth's surface. In general, sediments buried deeper and at higher temperatures than those that contain oil generate natural gas.
It is generally transported in bulk by pipeline in its natural gas form, or LNG carriers in its liquefied form; few countries transport it by truck.
Alternative sources
Apart from gas fields, an alternative method of obtaining methane is via biogas generated by the fermentation of organic matter including manure, wastewater sludge, municipal solid waste (including landfills), or any other biodegradable feedstock, under anaerobic conditions. Rice fields also generate large amounts of methane during plant growth. Methane hydrates/clathrates (ice-like combinations of methane and water on the sea floor, found in vast quantities) are a potential future source of methane. Cattle belch methane accounts for 16% of the world's annual methane emissions to the atmosphere.[48] One study reported that the livestock sector in general (primarily cattle, chickens, and pigs) produces 37% of all human-induced methane.[49] Early research has found a number of medical treatments and dietary adjustments that help slightly limit the production of methane in ruminants.[50][51] A more recent study, in 2009, found that at a conservative estimate, at least 51% of global greenhouse gas emissions were attributable to the life cycle and supply chain of livestock products, meaning all meat, dairy, and by-products, and their transportation.[52] Many efforts are underway to reduce livestock methane production and trap the gas to use as energy.[53]
Paleoclimatology research published in Current Biology suggests that flatulence from dinosaurs may have warmed the Earth.[54]
Atmospheric methane
Main article: Atmospheric methaneMethane is created near the Earth's surface, primarily by microorganisms by the process of methanogenesis. It is carried into the stratosphere by rising air in the tropics. Uncontrolled build-up of methane in the atmosphere is naturally checked – although human influence can upset this natural regulation – by methane's reaction with hydroxyl radicalsformed from singlet oxygen atoms and with water vapor. It has a net lifetime of about 10 years,[55] and is primarily removed by conversion to carbon dioxide and water.
In addition, there is a large (but unknown) amount of methane in methane clathrates in the ocean floors as well as the Earth's crust. Most methane is the result of biological process called methanogenesis.
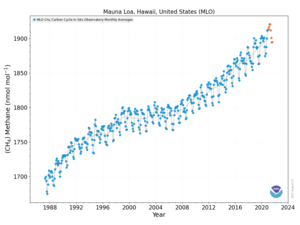
In 2010, methane levels in the Arctic were measured at 1850 nmol/mol, a level over twice as high as at any time in the 400,000 years prior to the industrial revolution. Historically, methane concentrations in the world's atmosphere have ranged between 300 and 400 nmol/mol during glacial periods commonly known as ice ages, and between 600 to 700 nmol/mol during the warm interglacial periods. Recent research suggests that the Earth's oceans are a potentially important new source of Arctic methane.[56]
Methane is an important greenhouse gas with a global warming potential of 34 compared to CO2 over a 100-year period, and 72 over a 20-year period.[57][58][59]
The Earth's atmospheric methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases (these gases don't include water vapor which is by far the largest component of the greenhouse effect).[6]
Clathrates
Methane is essentially insoluble in water, but it can be trapped in ice forming a similar solid. Significant deposits of methane clathrate have been found under sediments on the ocean floors of Earth at large depths.
Arctic methane release from permafrost and methane clathrates is an expected consequence and further cause of global warming.[60][61][62]
Anaerobic oxidation of methane
There is a group of bacteria that drive methane oxidation with nitrite as the oxidant, the anaerobic oxidation of methane.[63]
Safety
Methane is nontoxic, yet it is extremely flammable and may form explosive mixtures with air. Methane is violently reactive with oxidizers, halogen, and some halogen-containing compounds. Methane is also an asphyxiant and may displace oxygen in an enclosed space. Asphyxia may result if the oxygen concentration is reduced to below about 16% by displacement, as most people can tolerate a reduction from 21% to 16% without ill effects. The concentration of methane at which asphyxiation risk becomes significant is much higher than the 5–15% concentration in a flammable or explosive mixture. Methane off-gas can penetrate the interiors of buildings near landfills and expose occupants to significant levels of methane. Some buildings have specially engineered recovery systems below their basements to actively capture this gas and vent it away from the building.
Methane gas explosions are responsible for many deadly mining disasters.[64] A methane gas explosion was the cause of the Upper Big Branch coal mine disaster in West Virginia on April 5, 2010, killing 25.[65]
Extraterrestrial methane
Methane has been detected or is believed to exist on all planets of the solar system, as well as on most of the larger moons. In most cases, it is believed to have been created by abiotic processes. Possible exceptions are Mars and Titan.
- Mercury – the tenuous atmosphere contains trace amounts of methane.[66]
- Venus – the atmosphere contains a large amount of methane from 60 km (37 mi) to the surface according to data collected by the Pioneer Venus Large Probe NeutralMass Spectrometer[67]
- Moon – traces are outgassed from the surface[68]
- Mars – the Martian atmosphere contains 10 nmol/mol methane.[69] The source of methane on Mars has not been determined. Recent research suggests that methane may come from volcanoes, fault lines, or methanogens,[70] or that it may be a byproduct of electrical discharges from dust devils and dust storms,[71] or that it may be the result of UVradiation.[72] In January 2009, NASA scientists announced that they had discovered that the planet often vents methane into the atmosphere in specific areas, leading some to speculate this may be a sign of biological activity going on below the surface.[73] Analysis of observations made by a Weather Research and Forecasting model for Mars (MarsWRF) and related Mars general circulation model (MGCM) suggests that it is potentially possible to isolate methane plume source locations to within tens of kilometers, which is within the roving capabilities of future Mars rovers.[74] TheCuriosity rover, which landed on Mars in August 2012, is able to make measurements that distinguish between different isotopologues of methane;[75] but even if the mission is to determine that microscopic Martian life is the source of the methane, the life forms likely reside far below the surface, outside of the rover's reach.[76] Curiosity's Sample Analysis at Mars (SAM) instrument is capable of tracking the presence of methane over time to determine if it is constant, variable, seasonal, or random, providing further clues about its source.[77] The first measurements with the Tunable Laser Spectrometer (TLS) indicated that there is less than 5 ppb of methane at the landing site at the point of the measurement.[78][79][80][81] The Mars Trace Gas Mission orbiter planned to launch in 2016 would further study the methane,[82][83] as well as its decomposition products such as formaldehyde and methanol. Alternatively, these compounds may instead be replenished by volcanic or other geological means, such as serpentinization.[47] On July 19, 2013, NASA scientists reported finding "not much methane" (i.e., "an upper limit of 2.7 parts per billion of methane") around the Gale Crater area where the Curiosity rover landed in August 2012.[84][85][86] On September 19, 2013, NASA scientists, on the basis of further measurements by Curiosity, reported no detection of atmospheric methane with a measured value of 0.18±0.67 ppbv corresponding to an upper limit of only 1.3 ppbv (95% confidence limit) and, as a result, conclude that the probability of current methanogenic microbial activity on Mars is reduced.[87][88][89] On 16 December 2014, NASA reported the Curiosity rover detected a "tenfold spike", likely localized, in the amount of methane in the Martian atmosphere. Sample measurements taken "a dozen times over 20 months" showed increases in late 2013 and early 2014, averaging "7 parts of methane per billion in the atmosphere." Before and after that, readings averaged around one-tenth that level.[90][91]
- Saturn – the atmosphere contains 4500 ± 2000 ppm methane[92]
- Enceladus – the atmosphere contains 1.7% methane[93]
- Iapetus[citation needed]
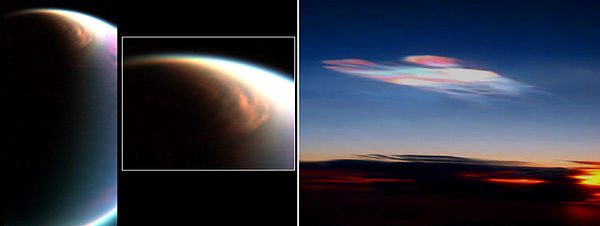
- Titan – the atmosphere contains 1.6% methane and thousands of methane lakes have been detected on the surface.[94] In the upper atmosphere the methane is converted into more complex molecules including acetylene, a process that also produces molecular hydrogen. There is evidence that acetylene and hydrogen are recycled into methane near the surface. This suggests the presence either of an exotic catalyst, or an unfamiliar form of methanogenic life.[95] Methane showers, probably prompted by changing seasons, have also been observed.[96] On October 24, 2014, methane was found in polar clouds on Titan.[97][98]
- Uranus – the atmosphere contains 2.3% methane[99]
- Ariel – methane is believed to be a constituent of Ariel's surface ice
- Miranda[citation needed]
- Oberon – about 20% of Oberon's surface ice is composed of methane-related carbon/nitrogen compounds
- Titania – about 20% of Titania's surface ice is composed of methane-related organic compounds[citation needed]
- Umbriel – methane is a constituent of Umbriel's surface ice
- Neptune – the atmosphere contains 1.5 ± 0.5% methane[100]
- Pluto – spectroscopic analysis of Pluto's surface reveals it to contain traces of methane[103][104]
- Eris – infrared light from the object revealed the presence of methane ice[106]
- Halley's Comet
- Comet Hyakutake – terrestrial observations found ethane and methane in the comet[107]
- Extrasolar planets – methane was detected on extrasolar planet HD 189733b; this is the first detection of an organic compound on a planet outside the solar system. Its origin is unknown, since the planet's high temperature (700 °C) would normally favor the formation of carbon monoxide instead.[108] Research indicates that meteoroidsslamming against exoplanet atmospheres could add organic gases such as methane, making the exoplanets look as though they are inhabited by life, even if they are not.[109]
- Interstellar clouds[110]
- The atmospheres of M-type stars.[111]
See also
- 2007 Zasyadko mine disaster
- Abiogenic petroleum origin
- Aerobic methane production
- Anaerobic digestion
- Anaerobic respiration
- Arctic methane release
- Biogas
- Coal Oil Point seep field
- Energy density
- Global Methane Initiative
- Greenhouse gas
- Halomethane, halogenated methane derivatives.
- Industrial gas
- Lake Kivu (more general: limnic eruption)
- List of alkanes
- Methanation
- Methane clathrate, ice that contains methane.
- Methane (data page)
- Methane on Mars: atmosphere
- Methane on Mars: climate
- Methanogen, archaea that produce methane.
- Methanogenesis, microbes that produce methane.
- Methanotroph, bacteria that grow with methane.
- Methyl group, a functional group related to methane.
- Organic gas
- Thomas Gold
Notes
- ^ There are many serpentinization reactions. Olivine is a solid solution between forsterite and fayalite whose general formula is (Fe,Mg)2SiO4. The reaction producing methane from olivine can be written as: Forsterite + Fayalite + Water + Carbonic acid → Serpentine + Magnetite + Methane , or (in balanced form): 18 Mg2SiO4 + 6 Fe2SiO4 + 26 H2O + CO2 → 12 Mg3Si2O5(OH)4 + 4 Fe3O4 + CH4
'과학과 테크놀로지 > 환경' 카테고리의 다른 글
(환경) 투명사회위한정보공개센터: <2013 탈바꿈프로젝트>원자력이 싸다고? 그럼 발전단가 산출근거부터 공개해! (0) 2016.01.10 (환경) Dirty, Dangerous and Expensive: The Truth About Nuclear Power (영문) (0) 2016.01.10 (환경) 메탄 가스 (0) 2016.01.09 (환경/미국) LA 메탄 가스 누출 사고: 이번 사고는 재앙이 아니라고 말하는 회사 (0) 2016.01.09 (환경) 억만장자에서 환경보호활동가 된 노스페이스 창립자 더글라스 톰킨스 (0) 2015.12.10